Chromatography is one of the most common methods for the purification of recombinant proteins, and more specifically affinity chromatography is the one that is mostly used due to its high specificity, which allows us to obtain great purity in one single step. When using this technique, a tag is added to the protein of interest, a small structure that is not included in the original protein and that allows us to easily capture it. As we explain in our protein purification handbook, tags are generally short sequences of 3-4 amino acids (up to a maximum of 15) and are intended to minimize as much as possible the properties of the protein.
We have to take into account that there are very sensitive proteins that easily lose their activity when their sequences are modified; therefore, this technique cannot be used for all cases. An initial study should be performed in order to determine in which region of the protein the tag will be incorporated, out of which the most common and less harmful are the terminal ends and solvent-exposed loops. In some cases, it is convenient to eliminate the tag after the purification in order to obtain a protein as similar as possible to the original; thus, there are enzymatic methods for this function. In other circumstances, if the tag doesn’t cause activity loss, there is no need to eliminate it and it can be left in the final structure.
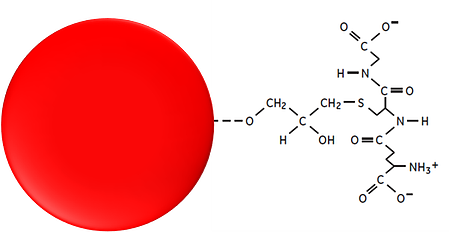
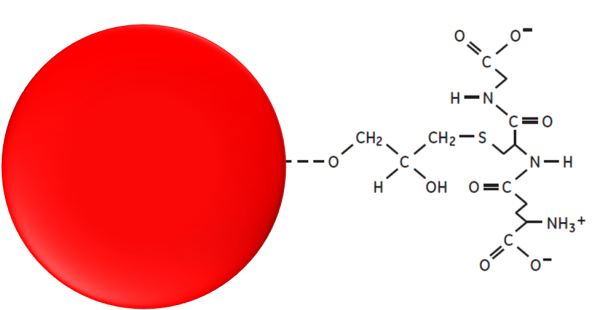
Hence, a tag allows us to separate the protein from the rest of the elements in the sample, but it also allows us to detect the presence of the protein in case there is no specific antibody for it. In this case, we add the protein, a known tag, and a specific antibody is used to measure its presence during the entire process. At the end, this turns out to be cheaper rather than producing a specific antibody for the protein, which is a very long and expensive process.
This post is an excerpt from our protein purification handbook, which explains the basics of recombinant protein purification. Download The Basic Guide to Recombinant Protein Purification here:
Which are the most popular tags for purification?
The most widely used tags in the purification of recombinant proteins are the histidine tags, which are incorporated either into the C- or N-terminal ends. They consist of a 6-histidine residue motif (at least), an amino acid that has high affinity and selectivity for the ions of nickel and other metals. These ions can be bound in a matrix through chelating agents with which they form complexes.
By this “tagging” process, a protein with the histidine tag is the molecule that presents a stronger binding with the matrix (in comparison to the rest of the molecules of a bacterial extract, for example). That allows us to obtain a relatively pure protein with only one purification step. If we add this effectiveness to the fact that it is a small tag (thus hardly disruptive) and that it only depends on the primary structure of the protein, it is easily understood why it is the preferred option.
Other common tags
Another popular option is Glutathione S-Transferase (GST) tags, which are based on the affinity of GST by the glutathione ligand (that can be bound to a matrix). This binding to the ligand is reversible, and allows a gentle purification process that doesn’t affect the protein structure or function. However, this tag has a larger size than the histidine one; thus, it may be difficult working with it.
We also have the Maltose Binding Protein (MBP) tag and the Strep-tag II. The first one binds to maltose and they can confer to the fused protein increased expression levels and higher solubility, additionally facilitating the protein folding. Regarding the Strep-tag II, it is a small sequence of 8 amino acids, which due to their size rarely interferes in the protein structure; therefore, there is no need to eliminate it. It binds specifically to the streptavidin ligand, which can also be bound in a matrix.
As we have seen, we have a great variety of tags for the purification of recombinant proteins. Although the histidine tags are the most widely used, the rest also offer many interesting advantages that we should consider.
Did you find this post interesting? You can check this related post in order to learn more about protein purification: