Magnetic bead separation enables complexes of magnetic beads and their bound materials to be separated from a complex mixture in solution with a single magnetic separation rack. The result is an isolated solution of your target biological elements which can be enriched and concentrated through this process.
A great deal of work goes into characterizing and parameterizing the process done by magnetic beads themselves. Users of magnetic beads need to know how to specify them by several factors including (but not limited to): their diameter, density, magnetic pigment content, surface activation (plain, covalent, bio-functionalized) and even the acceptable variations of these parameters.
Another important consideration for the magnetic bead separation technique is the magnetic separator which will separate your beads from solution.. A magnetic field from an external source is required to move the beads, which is usually called a magnetic separation rack. Biomagnetic separation processes are optimized by determining the best specifications for the magnetic beads and for the magnetic separation rack.
A key parameter which defines the biomagnetic separation process is magnetic force. There are two major forces that play in your solution.
- The drag force generation by the viscosity of your solution that keeps your molecules from separating.
- The magnetic force of your magnetic separator rack that overcomes the drag with magnetic force.
The most efficient magnetic bead separation occurs when the separation conditions (magnetic force) are homogenous throughout the working volume. Therefore, it is equally important to ensure that the separation rack can scale with the working volume. Many traditional separation racks are only effective for a few milliliters of solution, and are not suitable for magnetic separation of liters of solution, and do not effectively scale to larger volumes of solution. When the working volume varies between batches and the magnetic force is not homogeneous throughout the sample, significant problems are likely to occur with batch-to-batch consistency. This manifests in the form of long separation times, magnetic bead loss, and irreversible aggregation/clumping problems, among other problems.
This post is about magnetic bead separation and how to validate this process. If you are interested in this topic, and are interested in learning more about it, download our Free Guide: The Starting Guide to Validate Biomagnetic Separation Processes.
Magnetic bead separation systems: maintaining lot consistency
How can lot-to-lot consistency be ensured without a finely-tuned magnetic separator rack? How can in-lot consistency be ensured if we do not know if the magnetic bead separation conditions are homogenous in the working volume?
This problem exists when working with samples on a small scale, but can be masked by the variability of the magnetic beads themselves. It is when the magnetic bead separation process involves different working volumes (because validation is done at different scales or production volume is larger than the final application), that the problem arises due to long separation times, significant increases of magnetic beads (and biomolecules) losses and irreversible aggregation problems (clumps).
These problems are often no more than the consequences of lack of specification in the magnetic field profile used for the magnetic bead separation because, in most cases, nobody is even aware of the need to take this factor into account.
To avoid these issues, we simply need to ask ourselves the key parameter to define a biomagnetic separation process.
The answer is not too complex. The key parameter to define the biomagnetic separation process is magnetic force. Magnetic force determines the magnetic bead separation speed by overcoming opposition to the drag force generated by the viscosity of the buffer.
Magnetic force is the key parameter defining magnetic bead separation
The most basic concept of magnetic force is that homogenous magnetic fields do not generate magnetic force; they only generate magnetic torque. Regardless of the strength of the magnetic field, if there is no gradient, the beads do not move - they merely spin to align their magnetic moments with the field. This will not help to separate your biological materials!
To generate a magnetic force, a magnetic field that changes with distance (a magnetic field gradient) is required. A strong permanent magnet is often used for magnetic bead separation because it generates a strong magnetic field gradient, not because of the high value of the magnetic field.
A closer look at the formula shows that magnetic force depends on two variables:
- The magnetic moment of the beads
- The profile of the magnetic field
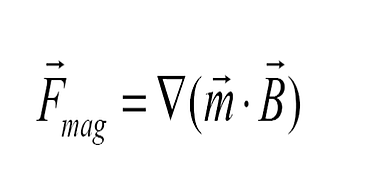
Depending on the magnetic behavior of the beads, magnetic moment changes linearly with the applied magnetic field (when susceptibility is constant) or, if the field is strong enough, their magnetic moment is constant (the bead is magnetically saturated).
By rewriting the magnetic force expression for each of the two magnetic behaviors, we found that the dynamics of the beads are different.
- If the magnetic bead has a magnetic moment that varies (i.e. a linear response with a constant susceptibility), the magnetic beads experience a force proportional to the gradient of the square of the magnetic field.
- However, when saturated (constant magnetic moment), the beads experience a force proportional to the gradient of the magnetic field.
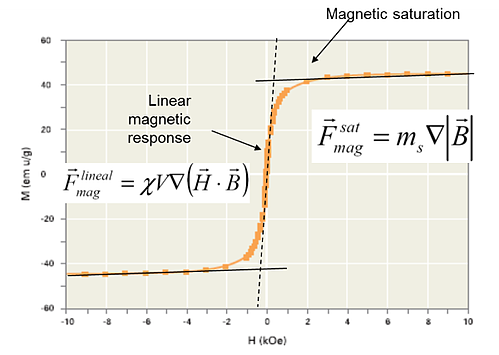
If we simultaneously fulfill requirements for the saturation of the magnetic material and a constant magnetic field gradient, we obtain a constant magnetic force (exactly what we achieve in our Sepmag Biomagnetic Separation Systems). Now, having defined the magnetic bead separation process, we can look more closely at the dynamics of the process.
Bead behavior in magnetic bead separation
The simplest assumption is that every magnetic bead moves independently. To test this case we first need to calculate the theoretical magnetic bead separation speed. If we know magnetic moment, magnetic field gradient, and buffer viscosity we can calculate the separation speed. Then we can predict the separation time for the farthest bead is obtained by simply dividing distance by speed.

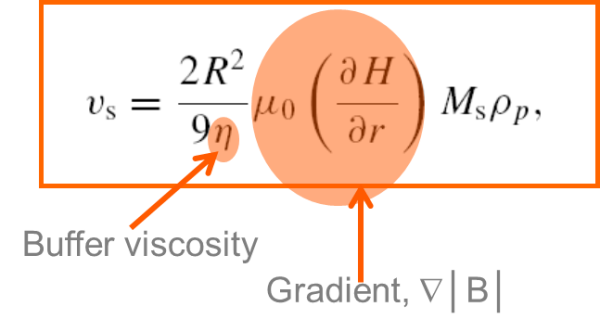
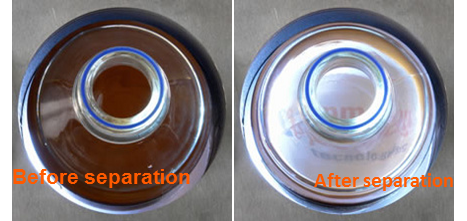
We can then test the formula by comparing experimental results. Using a cylindrical vessel and a Sepmag separation rack that generates a radial magnetic field gradient, we can measure the light traversing the suspension. As the separation proceeds, the solution will change from cloudy to clear as the magnetic particles travel down the magnetic field gradient and collect at the outer edges of the vessel.
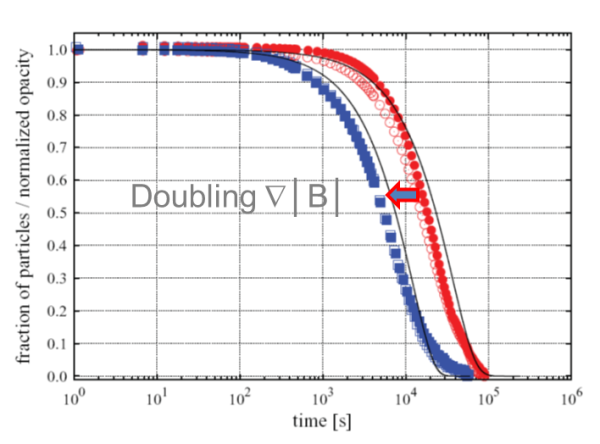
The experimental results below show very good agreement to the model for the small diameter magnetic particles used in this experiment. However, the magnetic bead separation time lasts one day! This theory correctly predicted the separation time and the effect of doubling the value of the magnetic field gradient, but these small magnetic beads would be impractical for the most life science applications that usually require separation times of a few seconds, such as CLIA immunoassays.
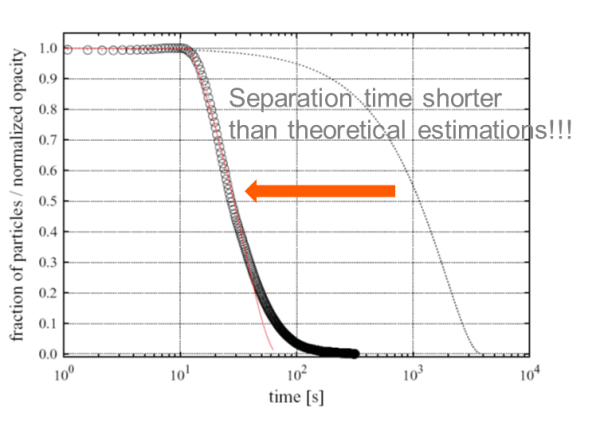
The same calculations can be done for suspensions with larger magnetic beads and using a similar magnetic field gradient. In this case, the predicted separation times are far shorter than experimental values! This experimental separation was complete in less than three minutes, which is dramatically faster than the predicted one-hour separation time.
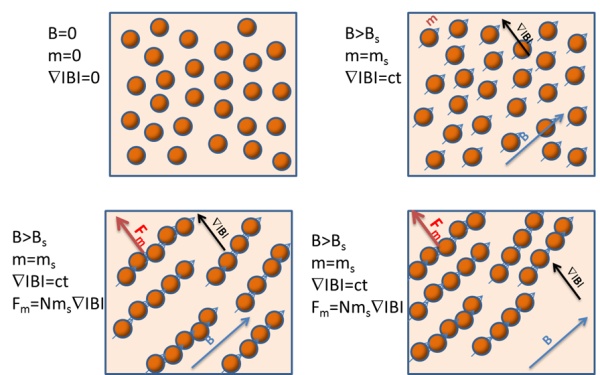
What is happening? The explanation is quite simple. The magnetic beads used in most life science applications do not behave like ‘’ particles.
Chain-like aggregation of magnetic beads improves separation time
When a magnetic field is applied to a suspension of magnetic beads of large enough diameter, the beads become magnetized and each one behaves like a small magnet, aligning with their neighbors and forming chain-like structures. These clusters move like very large ‘beads’, but with greater velocity than single magnetic beads. Note that, as the video shows, the chains are formed in the direction of the magnetic field, but the movement is along the magnetic field gradient direction.
If magnetic beads are superparamagnetic, once the magnetic field is removed (i.e. the vessel moved out of the biomagnetic separation device) their magnetic moment becomes zero and the chains dissolve.
This collective behavior has important practical consequences. As chain-like structures are key to the magnetic bead separation process, the concentration of the beads in suspension has an important impact on the separation time. Therefore, it is important to consider bead concentration when creating a magnetic bead separation protocol. The higher the concentration, the faster the magnetic bead separation: the closer the neighbor, the easier and quicker it is to form chains. [Ref 2]
Then, having defined the magnetic bead separation conditions, we have found that our beads can work in isolation or cooperatively. Separation times can change by several orders of magnitude depending on this, regardless of the magnetic separation rack used.
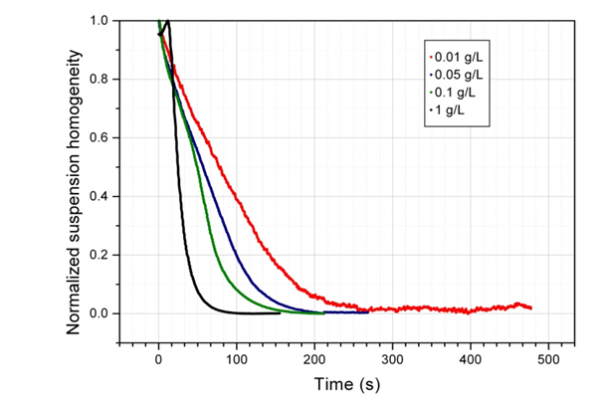
How do we know when magnetic beads are acting cooperatively? Using homogenous biomagnetic separation system and monitoring the process, UAB and ICMAB researchers have developed models and tested them in cooperation with Sepmag R&D staff. [Ref 3]
They have defined an expression for the average length of the chains, N*. If N* is greater than 1, this denotes cooperative behavior. If N* is well below 1, the magnetic beads separate as isolated particles.
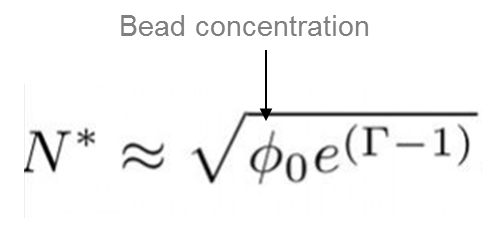
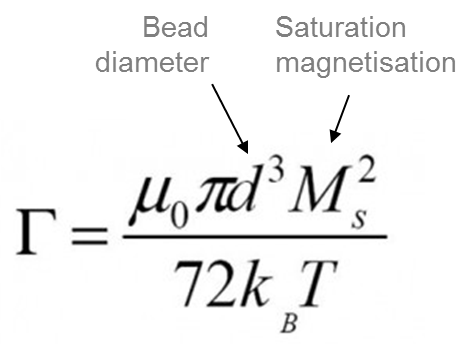
The value of N* is proportional to the square root of the concentration, but depends exponentially on, Γ, the ratio between the dipole-dipole magnetic energy and the thermal energy.
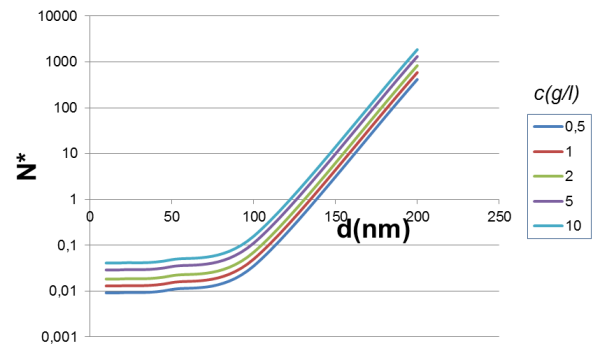
If we plot the value of N* according to the diameter for fixed magnetic pigment content, we see that by increasing the concentration we can collectively separate magnetic beads less big. The key parameter is bead size (note the logarithmic scale for the Y-axis). Increasing magnetic pigment content (or its magnetization) may be also a good strategy, but would also usually increase bead density and therefore the sedimentation ratio.
Too much sedimentation can be a problem if the beads precipitate to the bottom of the vessel before magnetic separation can occur.
In conclusion, magnetic bead separation is much more complicated than simply placing a permanent magnet next to a test tube. The magnetic moment of the beads and homogeneity of the magnetic field gradient are key parameters for ensuring separation speeds that are useful for bioscience applications. This is why choosing a properly scaled biomagnetic separation rack is crucial for ensuring batch-to-batch consistency. Additionally, the bead diameter and magnetic pigment concentration affect the behavior of the beads during the separation process and are equally important variables to consider when striving to achieve useful separation times. When sample sizes range from microliters to tens of liters, a cost-effective, rapid, consistent, and scalable process can only be achieved if the full magnetic bead separation process is well defined.

If you found this article interesting and want to get a deeper insight in the topic of magnetic bead separation, make sure to check these articles from our blog:
- The key to consistency: validation of biomagnetic separation processes
- The weakest link in IVD production
- The 6 key factors affecting the behavior of magnetic beads
REFERENCES:
- M. Benelmekki & Ll. M. Martinez “Magnetophoresis of iron oxide nanoparticles: A tool for synthesis monitoring and biomagnetic applications” "Drug Delivery and Nanomedicine" vol 5. Editor J.N. Govil, Studium Press LLC, USA (2013).
- J. Faraudo & J. Camacho (2010). Colloid Polym. Sci., 288:207
- J. S. Andreu et al, PHYSICAL REVIEW E 84, 021402 (2011)